Unlocking Reaction Secrets: Exploring Potential Energy Diagrams in Chemistry
Ever wondered how chemists predict the course of a chemical reaction? Imagine a roadmap for molecules transforming – that's essentially what a potential energy diagram provides in the realm of chemistry. These diagrams are powerful tools that visually represent the energy changes occurring throughout a chemical reaction, offering invaluable insights into reaction mechanisms, activation energies, and the overall energy landscape of a chemical transformation.
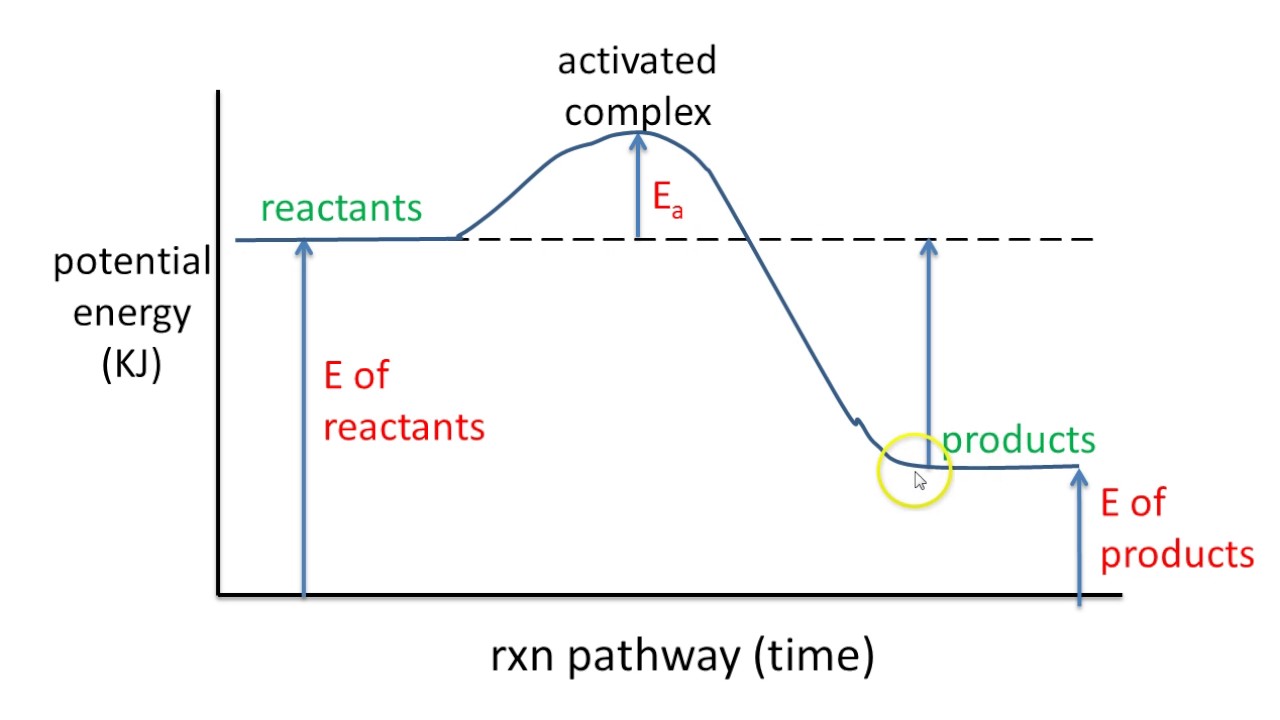
A chemical reaction isn't just a simple A-to-B transition. It's a journey with energy peaks and valleys. Potential energy diagrams, sometimes referred to as reaction coordinate diagrams, chart this journey, showing how the potential energy of the system changes as reactants transform into products. The x-axis, often labeled "reaction coordinate" or "reaction progress," represents the progression of the reaction from reactants to products. The y-axis represents the potential energy of the chemical system.
The concept of visualizing energy changes in chemical reactions emerged alongside the development of chemical kinetics and thermodynamics in the late 19th and early 20th centuries. Scientists like Svante Arrhenius contributed significantly to our understanding of reaction rates and the concept of activation energy, a key feature represented on potential energy diagrams. These diagrams became essential tools for chemists to interpret experimental data and build theoretical models of reaction mechanisms.
The central issue addressed by potential energy diagrams is understanding the energetic feasibility and pathway of a chemical reaction. Will a reaction occur spontaneously? How much energy is required to initiate the reaction? These questions, crucial for designing and controlling chemical processes, can be answered by analyzing the features of a potential energy diagram. For example, the difference in energy between the reactants and the highest energy point along the reaction pathway, known as the activation energy, determines the rate of the reaction.
A simple example is the reaction between hydrogen and oxygen to form water. The potential energy diagram for this reaction would show an initial energy level for the reactants (H₂ and O₂). As the reaction proceeds, the diagram shows an increase in potential energy, representing the energy required to break the bonds in the reactant molecules. This peak represents the transition state, a high-energy, unstable intermediate state. The diagram then shows a decrease in potential energy as new bonds form to create the product, water (H₂O). The difference between the energy of the reactants and the energy of the products represents the overall energy change of the reaction (enthalpy change).
One benefit of using these diagrams is the ability to visually compare different reaction pathways. Another advantage is the ability to understand the impact of catalysts. Catalysts lower the activation energy of a reaction, making it easier for the reaction to occur. This is reflected in the potential energy diagram by a lower energy peak for the catalyzed pathway compared to the uncatalyzed pathway. Finally, these diagrams provide a clear way to visualize exothermic (energy-releasing) and endothermic (energy-absorbing) reactions. In an exothermic reaction, the products are at a lower energy level than the reactants, while the opposite is true for an endothermic reaction.
Constructing a potential energy diagram typically involves plotting the potential energy of the system at various points along the reaction coordinate. This information can be obtained from computational chemistry calculations or experimental data. Key points to include are the energy levels of the reactants, products, and any intermediate states or transition states. The activation energy is determined by the difference between the energy of the reactants and the highest energy transition state.
Advantages and Disadvantages of Potential Energy Diagrams
| Advantages | Disadvantages |
|---|---|
| Visual representation of reaction pathways | Simplified representation of complex reactions |
| Clarifies activation energy and reaction mechanisms | Can be computationally intensive to generate accurate diagrams |
| Facilitates comparison of different reaction pathways | May not capture all the nuances of a real reaction system |
A common challenge in interpreting potential energy diagrams is understanding the difference between the transition state and an intermediate. The transition state is a fleeting, high-energy point, while an intermediate is a relatively stable species that exists for a longer duration during the reaction. Another challenge is accurately determining the activation energy, especially for complex reactions.
Frequently Asked Questions:
1. What does the peak on a potential energy diagram represent? (The transition state)
2. What is activation energy? (The minimum energy required to initiate a reaction)
3. How does a catalyst affect a potential energy diagram? (Lowers the activation energy)
4. What is the difference between an exothermic and an endothermic reaction on a potential energy diagram? (Product energy levels relative to reactant energy levels)
5. What is the reaction coordinate? (A measure of the progress of a reaction)
6. How are potential energy diagrams constructed? (Using computational or experimental data)
7. What is the significance of the transition state? (Represents the highest energy point along the reaction pathway)
8. How can I use a potential energy diagram to predict reaction rates? (By examining the activation energy)
In conclusion, the potential energy diagram in chemistry serves as an essential tool for understanding the intricacies of chemical reactions. By visualizing the energy changes that occur during a reaction, these diagrams provide invaluable information about reaction pathways, activation energies, and the influence of catalysts. From predicting reaction rates to designing new catalysts, understanding potential energy diagrams empowers chemists to control and manipulate chemical transformations. Explore further resources on chemical kinetics and thermodynamics to deepen your understanding of these powerful tools.
Renew insurance kereta malaysia dont get caught without it
Dive into the world of candy cigarettes manga a wild ride awaits
Unlocking brilliance with polar bear white paint from home depot